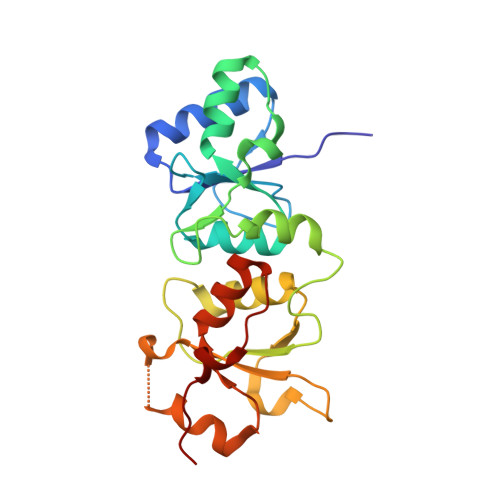
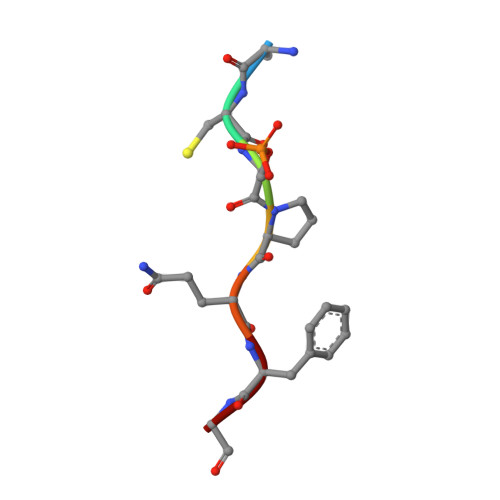
Structural Basis for the BRCA1 BRCT Interaction with the Proteins ATRIP and BAAT1.
Liu, X., Ladias, J.A.(2013) Biochemistry 52: 7618-7627
- PubMed: 24073851
- DOI: https://doi.org/10.1021/bi400714v
- Primary Citation of Related Structures:
4IFI, 4IGK - PubMed Abstract:
The breast and ovarian cancer susceptibility protein 1 (BRCA1) plays a central role in DNA damage response (DDR). Two tandem BRCA1 C-terminal (BRCT) domains interact with several proteins that function in DDR and contain the generally accepted motif pS-X-X-F (pS denoting phosphoserine and X any amino acid), including the ATR-interacting protein (ATRIP) and the BRCA1-associated protein required for ATM activation-1 (BAAT1). The crystal structures of the BRCA1 BRCTs bound to the phosphopeptides ATRIP (235-PEACpSPQFG-243) and BAAT1 (266-VARpSPVFSS-274) were determined at 1.75 Å and 2.2 Å resolution, respectively. The pSer and Phe(+3) anchor the phosphopeptides into the BRCT binding groove, with adjacent peptide residues contributing to the interaction. In the BRCA1-ATRIP structure, Gln(+2) is accommodated through a conformational change of the BRCA1 E1698 side chain. Importantly, isothermal titration calorimetry experiments showed that the size and charge of the side chains at peptide positions +1 and +2 contribute significantly to the BRCA1 BRCT-peptide binding affinity. In particular, the Asp(+1) and Glu(+2) in the human CDC27 peptide 816-HAAEpSDEF-823 abrogate the interaction with the BRCA1 BRCTs due in large part to electrostatic repulsion between Glu(+2) and E1698, indicating a preference of these domains for specific side chains at positions +1 and +2. These results emphasize the need for a systematic assessment of the contribution of the peptide residues surrounding pSer and Phe(+3) to the binding affinity and specificity of the BRCA1 BRCTs in order to elucidate the molecular mechanisms underlying the hierarchy of target selection by these versatile domains during DDR and tumorigenesis.
- Molecular Medicine Laboratory and Macromolecular Crystallography Unit, Department of Medicine, Harvard Medical School , Boston Massachusetts 02215, United States.
Organizational Affiliation: