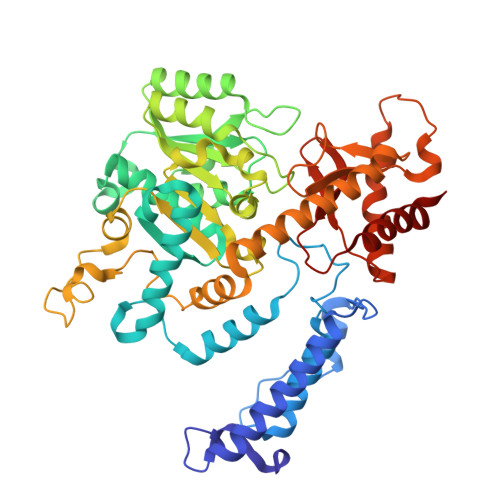
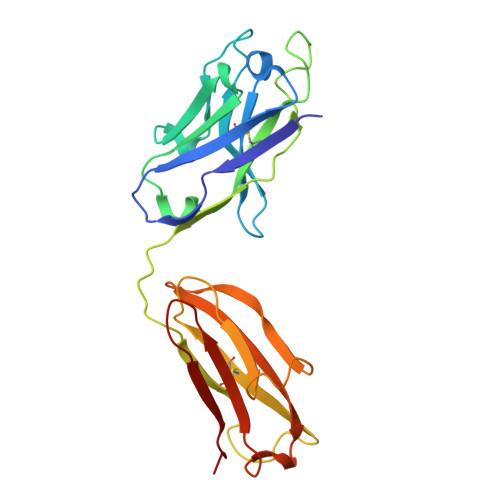
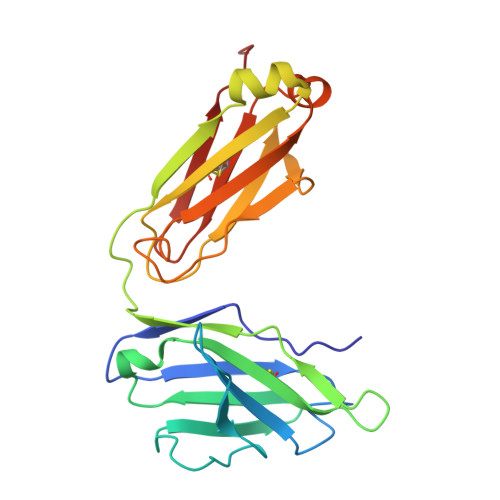
Structure and dynamics of GAD65 in complex with an autoimmune polyendocrine syndrome type 2-associated autoantibody.
Stander, S.H.D., Reboul, C.F., Le, S.N., Williams, D.E., Chandler, P.G., Costa, M.G.S., Hoke, D.E., Jimma, J.D.T., Fodor, J., Fenalti, G., Mannering, S.I., Porebski, B.T., Schofield, P., Christ, D., Buckle, M., McGowan, S., Elmlund, D., Rand, K.D., Buckle, A.M.(2025) Nat Commun 16: 2275-2275
- PubMed: 40055307
- DOI: https://doi.org/10.1038/s41467-025-57492-4
- Primary Citation of Related Structures:
7LZ6, 9D7Y - PubMed Abstract:
The enzyme glutamate decarboxylase (GAD) produces the neurotransmitter GABA, using pyridoxal-5'-phosphate (PLP). GAD exists as two isoforms, GAD65 and GAD67. Only GAD65 acts as a major autoantigen, frequently implicated in type 1 diabetes and other autoimmune diseases. Here we characterize the structure and dynamics of GAD65 and its interaction with the autoimmune polyendocrine syndrome type 2-associated autoantibody b96.11. Using hydrogen-deuterium exchange mass spectrometry (HDX), X-ray crystallography, cryo-electron microscopy, and computational approaches, we examine the conformational dynamics of apo- and holoGAD65 and the GAD65-autoantibody complex. HDX reveals local dynamics accompanying autoinactivation, with the catalytic loop promoting collective motions at the CTD-PLP domain interface. In the GAD65-b96.11 complex, heavy chain CDRs dominate the interaction, with a long CDRH3 bridging the GAD65 dimer via electrostatic interactions with the 260 PEVKEK 265 motif. This bridging links structural elements controlling GAD65's conformational flexibility to its autoantigenicity. Thus, intrinsic dynamics, rather than sequence differences within epitopes, appear to be responsible for the contrasting autoantigenicities of GAD65 and GAD67. Our findings elucidate the structural and dynamic factors that govern the varying autoantibody reactivities of GAD65 and GAD67, offering a revised rationale for the autoimmune response to GAD65.
- Protein Analysis Group, Department of Pharmacy, University of Copenhagen, Copenhagen, Denmark.
Organizational Affiliation: