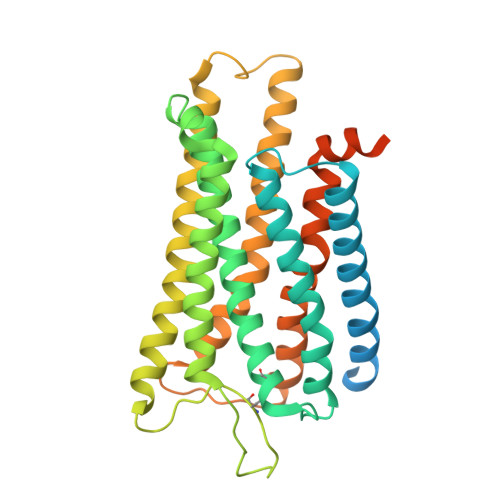
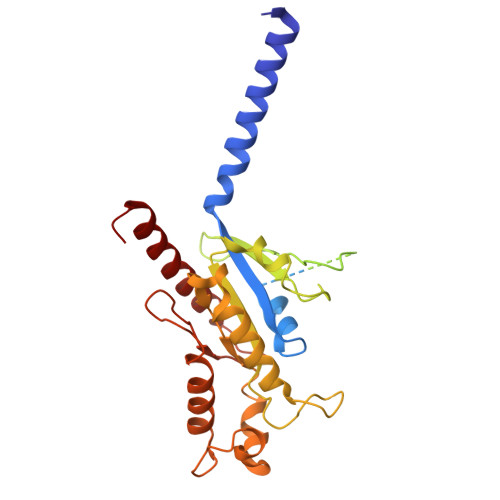
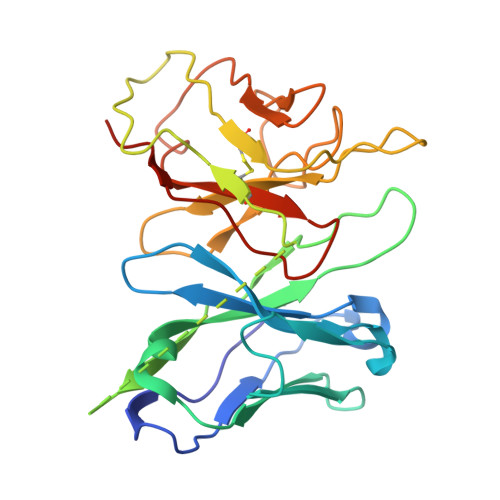
Molecular mechanisms of inverse agonism via kappa-opioid receptor-G protein complexes.
Tyson, A.S., Khan, S., Motiwala, Z., Han, G.W., Zhang, Z., Ranjbar, M., Styrpejko, D., Ramos-Gonzalez, N., Woo, S., Villers, K., Landaker, D., Kenakin, T., Shenvi, R., Majumdar, S., Gati, C.(2025) Nat Chem Biol 21: 1046-1057
- PubMed: 39775170
- DOI: https://doi.org/10.1038/s41589-024-01812-0
- Primary Citation of Related Structures:
8VVE, 8VVF, 8VVG, 9D61 - PubMed Abstract:
Opioid receptors, a subfamily of G protein-coupled receptors (GPCRs), are key therapeutic targets. In the canonical GPCR activation model, agonist binding is required for receptor-G protein complex formation, while antagonists prevent G protein coupling. However, many GPCRs exhibit basal activity, allowing G protein association without an agonist. The pharmacological impact of agonist-free receptor-G protein complexes is poorly understood. Here we present biochemical evidence that certain κ-opioid receptor (KOR) inverse agonists can act via KOR-G i protein complexes. To investigate this phenomenon, we determined cryo-EM structures of KOR-G i protein complexes with three inverse agonists: JDTic, norBNI and GB18, corresponding to structures of inverse agonist-bound GPCR-G protein complexes. Remarkably, the orthosteric binding pocket resembles the G protein-free 'inactive' receptor conformation, while the receptor remains coupled to the G protein. In summary, our work challenges the canonical model of receptor antagonism and offers crucial insights into GPCR pharmacology.
- The Bridge Institute, Michelson Center for Convergent Biosciences, University of Southern California, Los Angeles, CA, USA.
Organizational Affiliation: