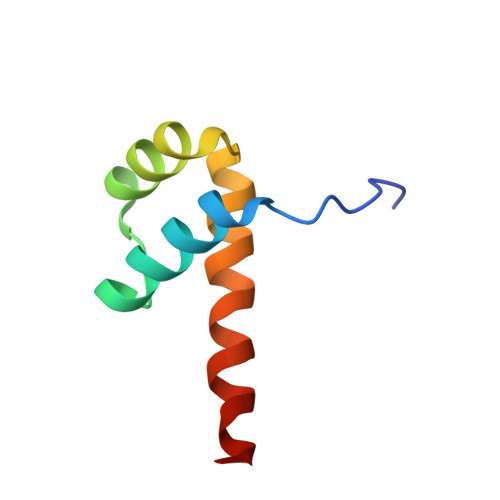
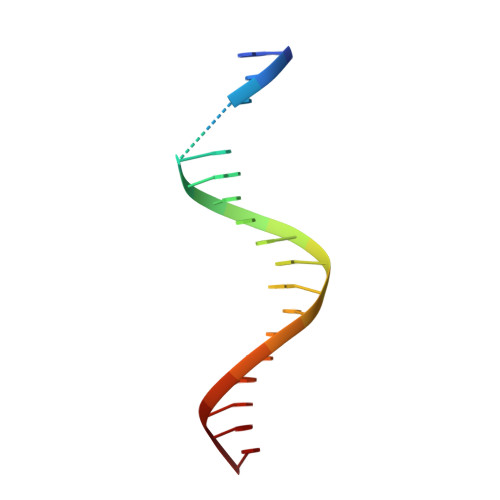
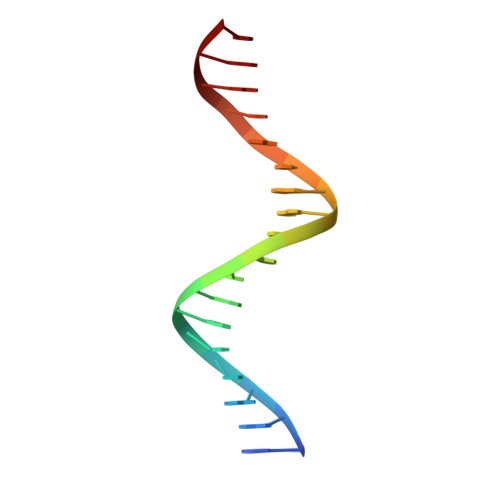
DNA-guided transcription factor interactions extend human gene regulatory code.
Xie, Z., Sokolov, I., Osmala, M., Yue, X., Bower, G., Pett, J.P., Chen, Y., Wang, K., Cavga, A.D., Popov, A., Teichmann, S.A., Morgunova, E., Kvon, E.Z., Yin, Y., Taipale, J.(2025) Nature
- PubMed: 40205063
- DOI: https://doi.org/10.1038/s41586-025-08844-z
- Primary Citation of Related Structures:
5EG0, 5NO6, 8BYX, 8BZM, 8R7F, 8R7Z - PubMed Abstract:
In the same way that the mRNA-binding specificities of transfer RNAs define the genetic code, the DNA-binding specificities of transcription factors (TFs) form the molecular basis of the gene regulatory code 1,2 . The human gene regulatory code is much more complex than the genetic code, in particular because there are more than 1,600 TFs that commonly interact with each other. TF-TF interactions are required for specifying cell fate and executing cell-type-specific transcriptional programs. Despite this, the landscape of interactions between DNA-bound TFs is poorly defined. Here we map the biochemical interactions between DNA-bound TFs using CAP-SELEX, a method that can simultaneously identify individual TF binding preferences, TF-TF interactions and the DNA sequences that are bound by the interacting complexes. A screen of more than 58,000 TF-TF pairs identified 2,198 interacting TF pairs, 1,329 of which preferentially bound to their motifs arranged in a distinct spacing and/or orientation. We also discovered 1,131 TF-TF composite motifs that were markedly different from the motifs of the individual TFs. In total, we estimate that the screen identified between 18% and 47% of all human TF-TF motifs. The novel composite motifs we found were enriched in cell-type-specific elements, active in vivo and more likely to be formed between developmentally co-expressed TFs. Furthermore, TFs that define embryonic axes commonly interacted with different TFs and bound to distinct motifs, explaining how TFs with a similar specificity can define distinct cell types along developmental axes.
- State Key Laboratory of Cardiovascular Diseases and Medical Innovation Center, Shanghai East Hospital, School of Medicine, Tongji University, Shanghai, China.
Organizational Affiliation: