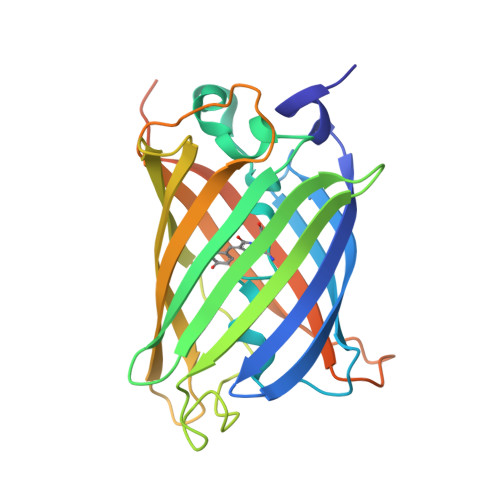
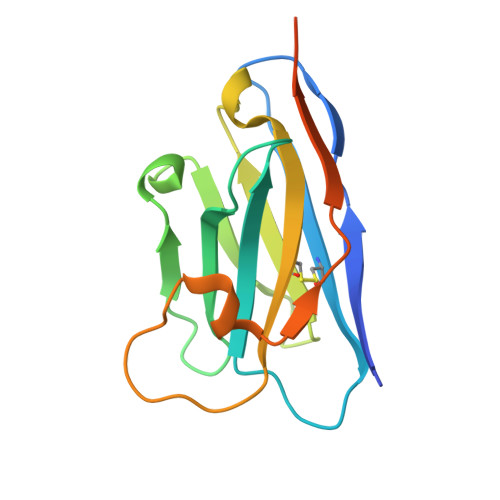
Unique mechanisms to increase structural stability and enhance antigen binding in nanobodies.
Ketaren, N.E., Fridy, P.C., Malashkevich, V., Sanyal, T., Brillantes, M., Thompson, M.K., Oren, D.A., Bonanno, J.B., Sali, A., Almo, S.C., Chait, B.T., Rout, M.P.(2025) Structure 33: 677-690.e5
- PubMed: 39938509
- DOI: https://doi.org/10.1016/j.str.2025.01.019
- Primary Citation of Related Structures:
8G0I, 8SFS, 8SFV, 8SFX, 8SFZ, 8SG3, 8SLC - PubMed Abstract:
Nanobodies are single domain antibody variants proving themselves to be compelling tools for research, disease diagnostics, and as therapeutics targeting a myriad of disease agents. However, despite this potential, their mechanisms of paratope presentation and structural stabilization have not been fully explored. Here, we show that unlike monoclonal antibodies, a nanobody repertoire maximizes sampling of an antigen surface by binding a single antigen in at least three different orientations, which are correlated with their paratope composition. Structure-guided reengineering of several nanobodies reveals that a single point mutation within the paratope or a highly conserved region of a nanobody's framework 3 (FR3) can markedly improve antigen affinity, nanobody stability, or both. Conversely, we show the negative impact on antigen affinity when "over-stabilizing" nanobodies. Collectively our results provide a universal strategy to tune a nanobody's affinity by modifying specific residues that can readily be applied to guide nanobody optimization and functionalization.
- Laboratory of Cellular and Structural Biology, The Rockefeller University, New York, NY 10065, USA. Electronic address: nketaren@rockefeller.edu.
Organizational Affiliation: