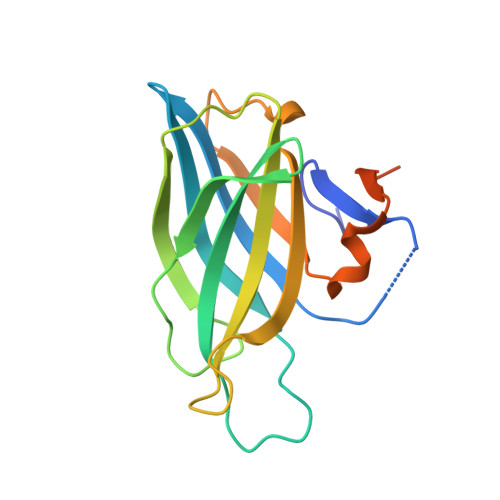
The liprin-alpha /RIM complex regulates the dynamic assembly of presynaptic active zones via liquid-liquid phase separation.
Jin, G., Campos, J., Liu, Y., de la Cruz, B.M., Zhang, S., Liang, M., Li, K., Xie, X., Sterky, F.H., Acuna, C., Wei, Z.(2025) PLoS Biol 23: e3002817-e3002817
- PubMed: 40493522
- DOI: https://doi.org/10.1371/journal.pbio.3002817
- Primary Citation of Related Structures:
8Z22 - PubMed Abstract:
Presynaptic scaffold proteins, including liprin-α, RIM, and ELKS, are pivotal to the assembly of the active zone and regulating the coupling of calcium signals and neurotransmitter release, yet the underlying mechanism remains poorly understood. Here, we determined the crystal structure of the liprin-α2/RIM1 complex, revealing a multifaceted intermolecular interaction that drives the liprin-α/RIM assembly. Neurodevelopmental disease-associated mutations block the formation of the complex. Disrupting this interaction in cultured human neurons impairs synaptic transmission and reduces the readily releasable pool of synaptic vesicles. Super-resolution imaging analysis supports a role for liprin-α in recruiting RIM1 to the active zone, presumably by promoting the liquid-liquid phase separation (LLPS) of RIM1. Strikingly, the liprin-α/RIM interaction modulates the competitive distribution of ELKS1 and voltage-gated Ca2+ channels (VGCCs) in RIM1 condensates. Disrupting the liprin-α/RIM interaction significantly decreased VGCC accumulation in the condensed phase and rendered release more sensitive to the slow calcium buffer EGTA, suggesting an increased physical distance between VGCC and vesicular calcium sensors. Together, our findings provide a plausible mechanism of the liprin-α/RIM complex in regulating the coupling of calcium channels and primed synaptic vesicles via LLPS for efficient synaptic transmission and uncover the pathological implication of liprin-α mutations in neurodevelopmental disorders.
- Shenzhen Key Laboratory of Biomolecular Assembling and Regulation, Southern University of Science and Technology, Shenzhen, China.
Organizational Affiliation: