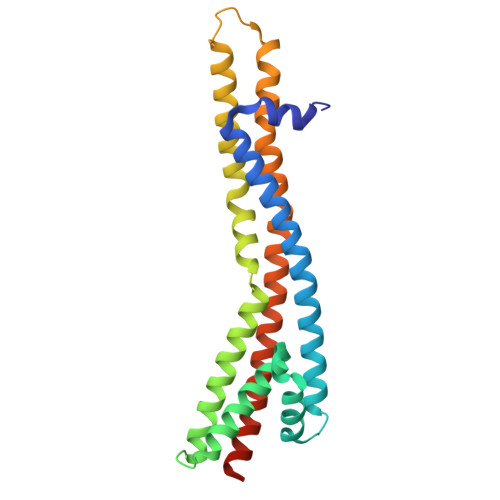
Cryo-EM structures of the E. coli Ton and Tol motor complexes.
Celia, H., Botos, I., Ghirlando, R., Duche, D., Beach, B.M., Lloubes, R., Buchanan, S.K.(2025) Nat Commun 16: 5506-5506
- PubMed: 40595649
- DOI: https://doi.org/10.1038/s41467-025-61286-z
- Primary Citation of Related Structures:
9DDM, 9DDN, 9DDO, 9DDP, 9DDQ - PubMed Abstract:
The Ton and Tol motor proteins use the proton gradient at the inner membrane of Gram-negative bacteria as an energy source. The generated force is transmitted through the periplasmic space to protein components associated with the outer membrane, either to maintain the outer membrane integrity for the Tol system, or to allow essential nutrients to enter the cell for Ton. We have solved the high-resolution structures of the E. coli TonB-ExbB-ExbD and TolA-TolQ-TolR complexes, revealing the inner membrane embedded engine parts of the Ton and Tol systems, and showing how TonB and TolA interact with the ExbBD and TolQR subcomplexes. Structural similarities between the two motor complexes suggest a common mechanism for the opening of the proton channel and the propagation of the proton motive force into movement of the TonB and TolA subunits. Because TonB and TolA bind at preferential ExbB or TolQ subunits, we propose a new mechanism of assembly of TonB and TolA with their respective ExbBD and TolQR subcomplexes and discuss its impact on the mechanism of action for the Ton and Tol systems.
- Laboratory of Molecular Biology, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, 20892, USA.
Organizational Affiliation: