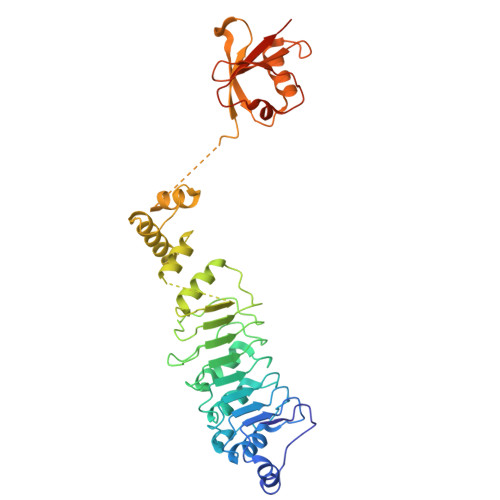
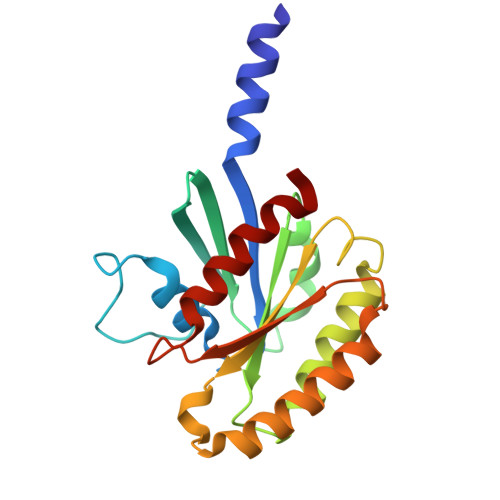
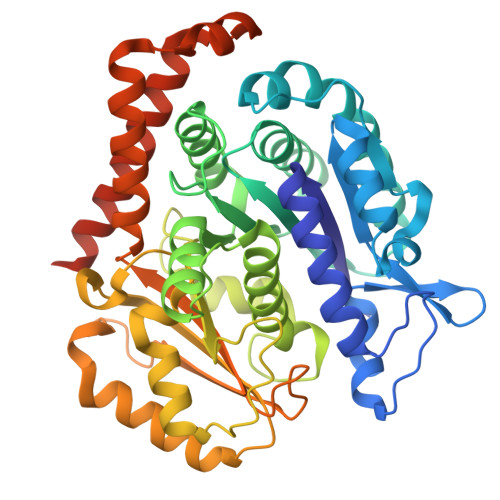
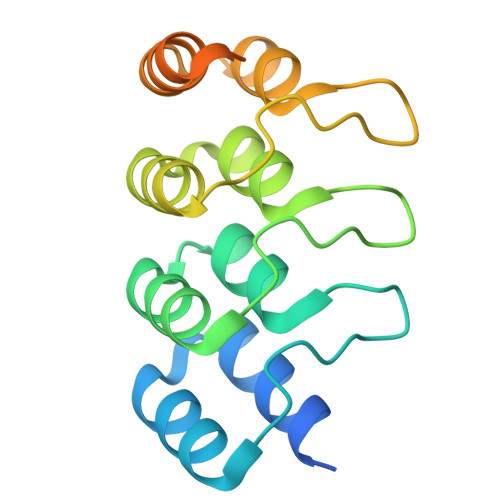
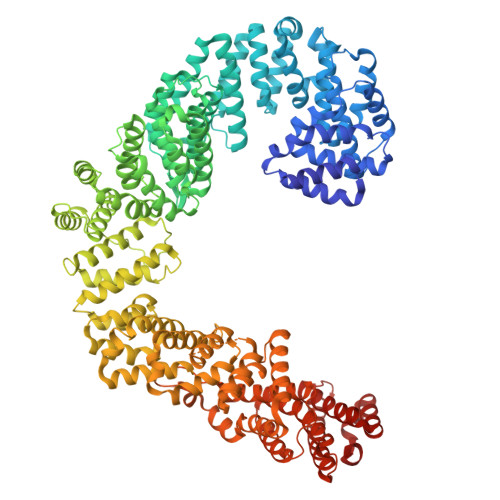
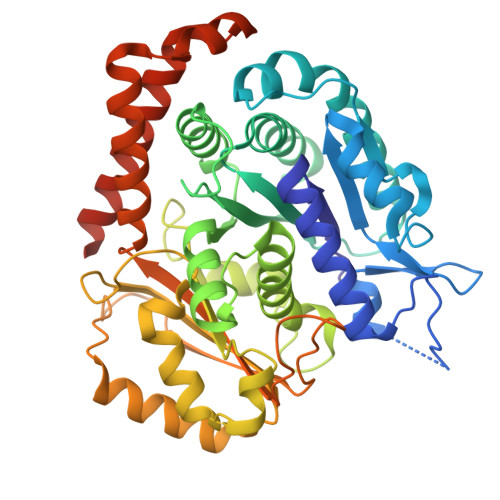
Cryo-EM structures of the tubulin cofactors reveal the molecular basis of alpha/beta-tubulin biogenesis.
Taheri, A., Wang, Z., Singal, B., Guo, F., Al-Bassam, J.(2025) Nat Commun 17: 1405-1405
- PubMed: 41461644
- DOI: https://doi.org/10.1038/s41467-025-68142-0
- Primary Citation of Related Structures:
9EDR, 9EDS, 9EDT, 9EEB - PubMed Abstract:
Microtubule polarity and dynamic polymerization arise from the self-association properties of the αβ-tubulin heterodimer. For decades, it has remained unclear how the tubulin cofactors TBCD, TBCE, TBCC, and the Arl2 GTPase mediate the biogenesis of αβ-tubulin from individual α- and β-tubulins. Here, we use cryo-electron microscopy to determine structures of tubulin cofactors bound to αβ-tubulin. TBCD, TBCE, and Arl2 form a heterotrimeric cage-like assembly, we term TBC-DEG, around the αβ-tubulin heterodimer. The TBC-DEG-αβ-tubulin structures show that TBC-DEG wraps around β-tubulin while TBCE extends along α-tubulin. The TBC-DEG/TBCC-αβ-tubulin structures reveal that TBCC forms multi-domain interactions with Arl2 and TBCD to engage the αβ-tubulin intradimer-interface, promoting TBCE rotation while TBCD holds β-tubulin. TBCC engages the GTP-bound Arl2, multiple sites of TBCD, and the native αβ-tubulin intradimer interface near the α-tubulin N-site GTP. Together, these structures uncover transition states for αβ-tubulin biogenesis and degradation, suggesting a vise-like, GTP-hydrolysis-dependent mechanism in which TBCC binding to TBC-DEG modulates αβ-tubulin interfaces. Our studies provide structural evidence that tubulin cofactors act as enzymatic regulators that assemble the invariant αβ-tubulin architecture. By catalyzing α- and β-tubulin biogenesis and degradation, the TBC-DEG and TBCC assemblies regulate the polymerization competency of αβ-tubulin for microtubule formation.
- Molecular Cellular Biology Department, University of California, Davis, CA, USA.
Organizational Affiliation: