Integrin alpha-v beta-3 in complex with rhodostomin
Wang, Y.T., Chuang, W.J.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Integrin alpha-V,Integrin alpha-V,Uncharacterized protein DKFZp686C11235 | 1,184 | Homo sapiens | Mutation(s): 0 Gene Names: ITGAV, MSK8, VNRA, VTNR, DKFZp686C11235 | 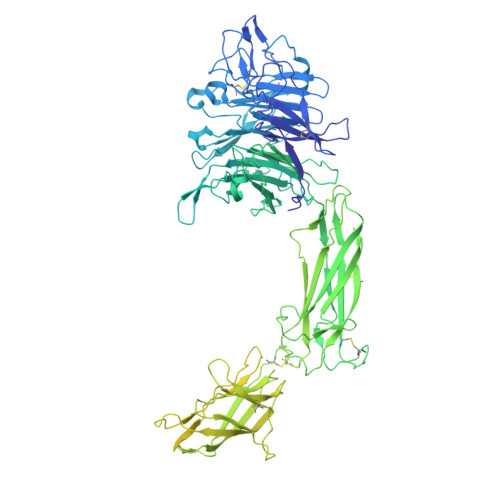 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P06756 (Homo sapiens) Explore P06756 Go to UniProtKB: P06756 | |||||
PHAROS: P06756 GTEx: ENSG00000138448 | |||||
Find proteins for Q6MZV7 (Homo sapiens) Explore Q6MZV7 Go to UniProtKB: Q6MZV7 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Groups | P06756Q6MZV7 | ||||
Glycosylation | |||||
| Glycosylation Sites: 6 | Go to GlyGen: P06756-1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Integrin beta-3,Integrin beta-3,Uncharacterized protein DKFZp686C11235 | 919 | Homo sapiens | Mutation(s): 0 Gene Names: ITGB3, GP3A, DKFZp686C11235 | 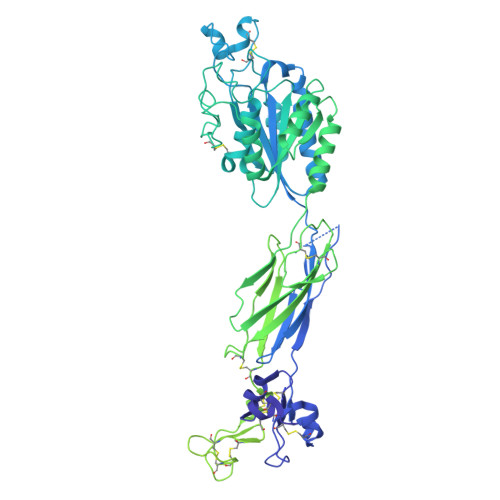 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P05106 (Homo sapiens) Explore P05106 Go to UniProtKB: P05106 | |||||
PHAROS: P05106 GTEx: ENSG00000259207 | |||||
Find proteins for Q6MZV7 (Homo sapiens) Explore Q6MZV7 Go to UniProtKB: Q6MZV7 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Groups | P05106Q6MZV7 | ||||
Glycosylation | |||||
| Glycosylation Sites: 3 | Go to GlyGen: P05106-1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Disintegrin rhodostomin | 68 | Calloselasma rhodostoma | Mutation(s): 0 | 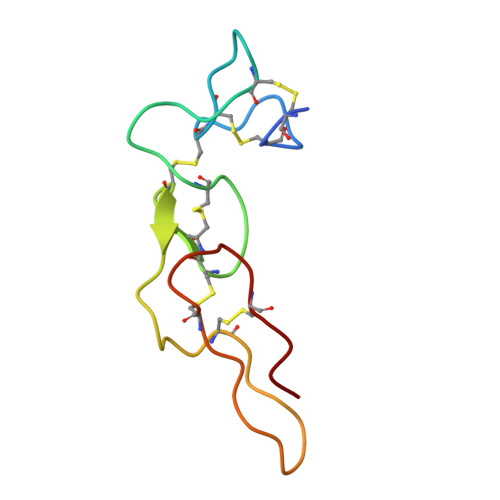 | |
UniProt | |||||
Find proteins for P30403 (Calloselasma rhodostoma) Explore P30403 Go to UniProtKB: P30403 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P30403 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 4 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | 2D Diagram | Glycosylation | 3D Interactions |
| beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose | D, G | 3 |  | N-Glycosylation | |
Glycosylation Resources | |||||
GlyTouCan: G15407YE GlyCosmos: G15407YE GlyGen: G15407YE | |||||
Entity ID: 5 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | 2D Diagram | Glycosylation | 3D Interactions |
| 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose | E, H, I [auth J], J [auth K] | 2 |  | N-Glycosylation | |
Glycosylation Resources | |||||
GlyTouCan: G42666HT GlyCosmos: G42666HT GlyGen: G42666HT | |||||
Entity ID: 6 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | 2D Diagram | Glycosylation | 3D Interactions |
| alpha-D-mannopyranose-(1-4)-beta-D-mannopyranose-(1-6)-[alpha-D-mannopyranose-(1-3)]beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose | F | 6 |  | N-Glycosylation | |
Glycosylation Resources | |||||
GlyTouCan: G93290PO GlyCosmos: G93290PO GlyGen: G93290PO | |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| NAG Query on NAG | P [auth A], T [auth B] | 2-acetamido-2-deoxy-beta-D-glucopyranose C8 H15 N O6 OVRNDRQMDRJTHS-FMDGEEDCSA-N |  | ||
| CA (Subject of Investigation/LOI) Query on CA | K [auth A] L [auth A] M [auth A] N [auth A] O [auth A] | CALCIUM ION Ca BHPQYMZQTOCNFJ-UHFFFAOYSA-N |  | ||
| MG (Subject of Investigation/LOI) Query on MG | Q [auth B] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | 1.20.1_4487: |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Science Council (NSC, Taiwan) | Taiwan | -- |