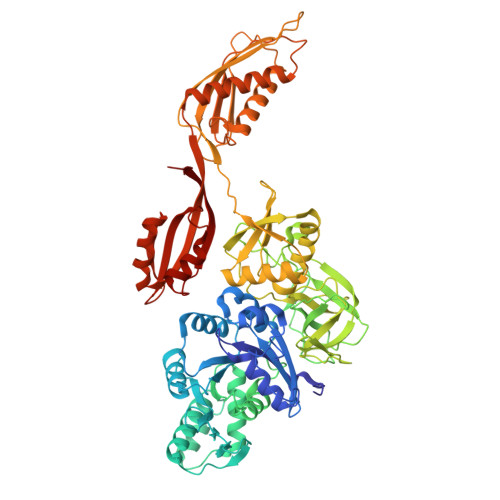
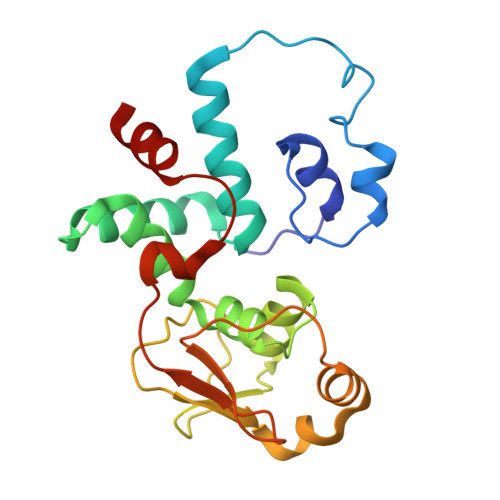
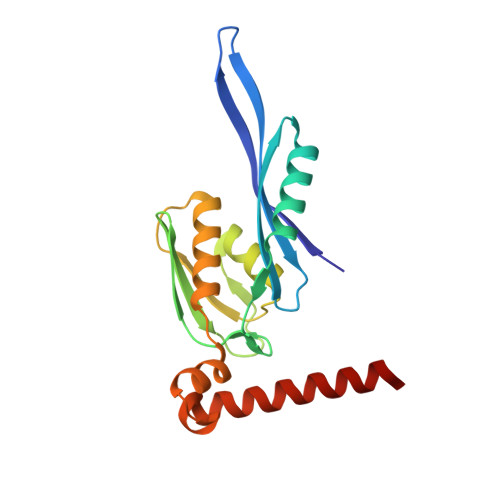
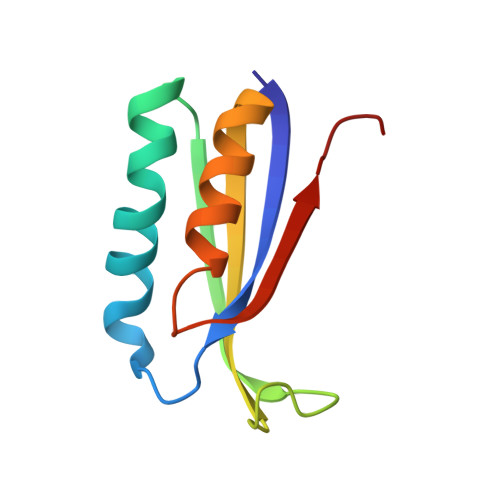
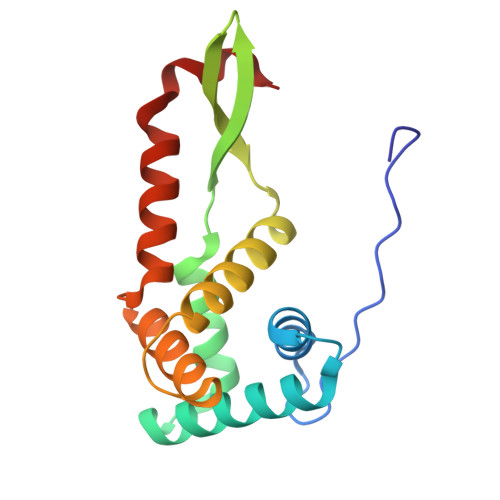
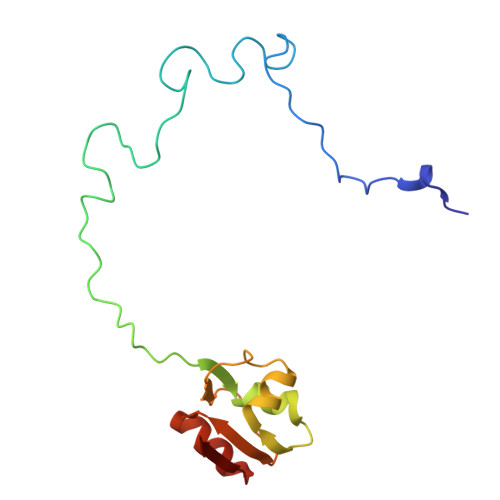
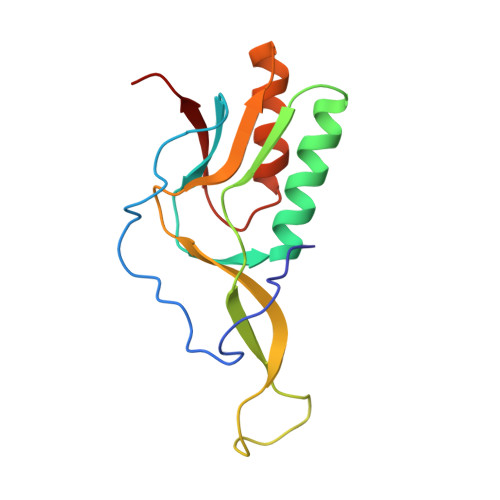
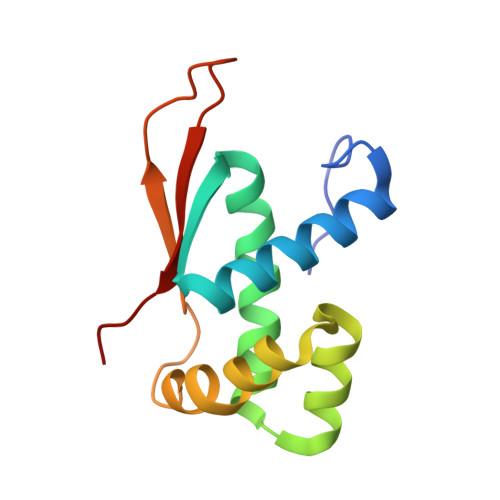
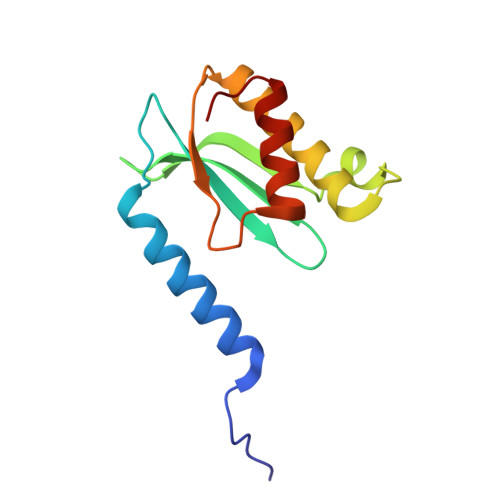
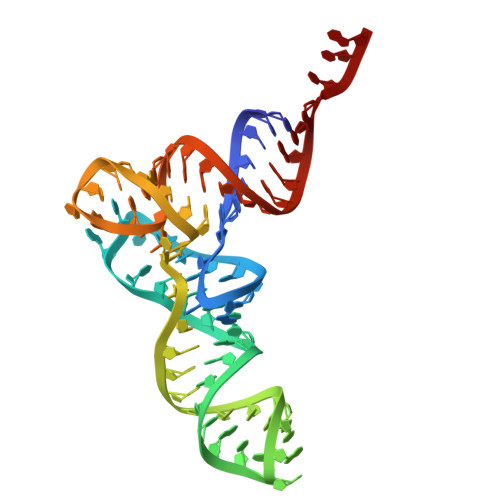
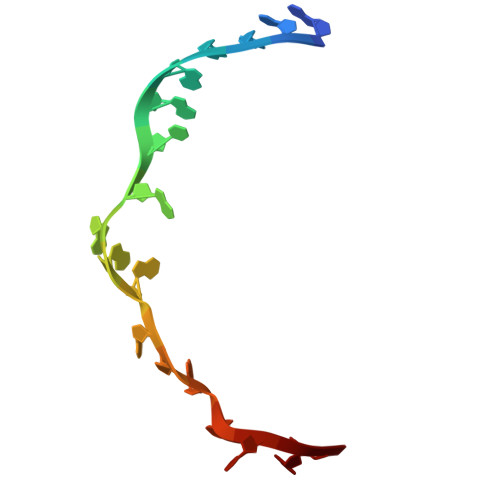
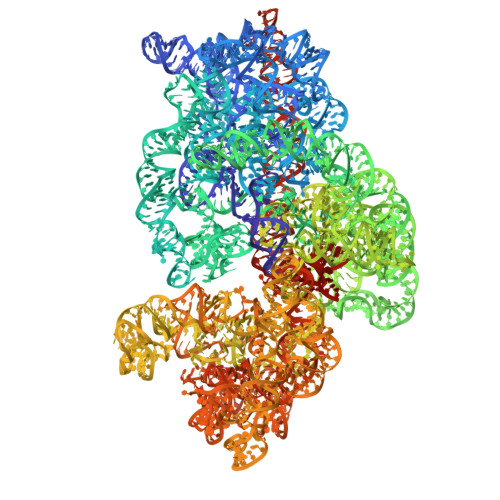
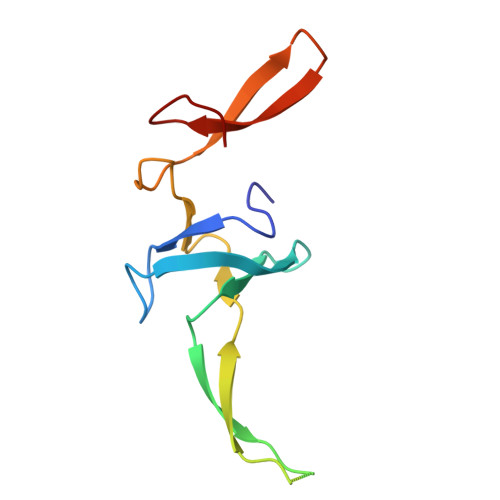Cryo-EM structural analyses reveal a unique role for elongation factor G2 (EF-G2) in Mycobacteria.
Baid, P., Sengupta, J.(2025) FEBS J
- PubMed: 40569974
- DOI: https://doi.org/10.1111/febs.70161
- Primary Citation of Related Structures:
9K0Z, 9K10 - PubMed Abstract:
The gene-encoding translation elongation factor G (EF-G) has undergone gene duplication across various bacterial species including Mycobacteria, and in mammalian mitochondria, leading to the emergence of the paralogue elongation factor G2 (EF-G2). Our study reveals that mycobacterial EF-G2, unlike EF-G1, neither participates in ribosome-recycling nor significantly contributes to overall translation, suggesting that it plays an alternative role in Mycobacteria. Remarkably, our investigation found a significant overexpression of mycobacterial EF-G2 during the stationary growth phase. Moreover, EF-G2 lacks ribosome-dependent GTPase activity, an observation consistent with previous reports. Cryo-EM analysis of the M. smegmatis 70S ribosome purified from the nutrient-starved (stationary) phase and complexed with EF-G2 unveiled the structural basis for its inability to hydrolyse GTP in a ribosome-dependent manner. Furthermore, we report an unprecedented binding mode of two EF-G2 copies on the 50S ribosomal subunit that impedes subunit association, thereby preventing the formation of active 70S ribosomes. Thus, instead of performing canonical functions, mycobacterial EF-G2 acts as a translation repressor during nutrient starvation. Altogether, our findings shed light on the multifaceted mechanisms by which EF-G2 modulates protein synthesis under nutrient-limited conditions, providing insights into adaptive strategies employed by Mycobacteria to survive in hostile environments.
- Structural Biology and Bioinformatics Division, CSIR-Indian Institute of Chemical Biology, Kolkata, India.
Organizational Affiliation: