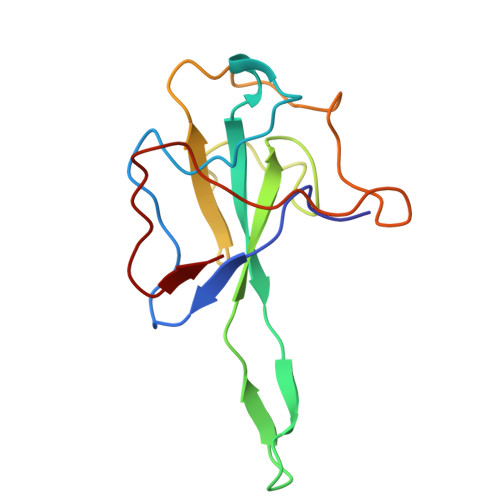
Structural insights into nucleocapsid protein variability: Implications for PJ34 efficacy against SARS-CoV-2.
Yamamoto, A., Ito, H., Sakaguchi, T., Higashiura, A.(2025) Virology 604: 110411-110411
- PubMed: 39848104
- DOI: https://doi.org/10.1016/j.virol.2025.110411
- Primary Citation of Related Structures:
9KN1 - PubMed Abstract:
Human coronaviruses (HCoVs) include common cold viruses such as HCoV-229E, OC43, NL63 and HKU1 as well as MERS-CoV and SARS-CoV, which cause severe respiratory disease. Recently, SARS-CoV-2 caused a COVID-19 pandemic. The nucleocapsid (N) protein of coronaviruses, which is essential for RNA binding and homodimerization, has a highly conserved structure across viruses. Previous studies revealed that compound PJ34 is an inhibitor of nucleic acid binding to the N-terminal domain (NTD) of the HCoV-OC43 N protein, suggesting that it could block viral replication. However, testing with SARS-CoV-2 showed that PJ34 did not inhibit viral replication. Structural analysis suggests that the substitution of Tyr for Ala at position 50 (corresponding to Tyr63 in OC43), may affect the ability to interact with compounds such as PJ34, explaining its lack of efficacy. These findings underscore the importance of structure-based drug development targeting the N protein, which remains an important therapeutic target in all coronaviruses.
- Department of Virology, Graduate School of Biomedical and Health Sciences, Hiroshima University, 1-2-3 Kasumi, Minami-ku, Hiroshima, 734-8551, Japan. Electronic address: akm@hiroshima-u.ac.jp.
Organizational Affiliation: