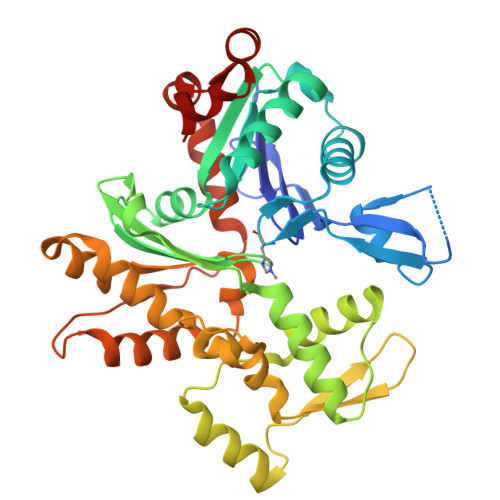
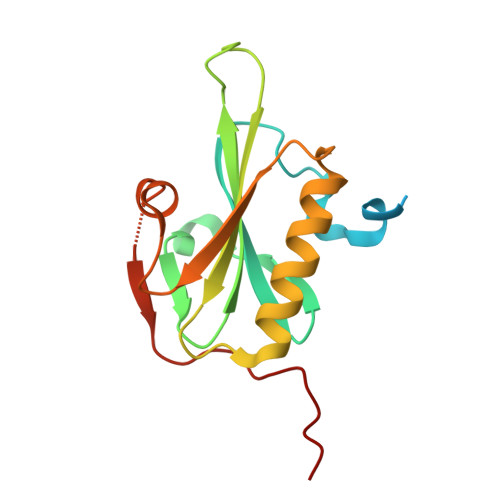
Microscopic and structural observations of actin filament capping and severing by cytochalasin D.
Mitani, T., Takeda, S., Oda, T., Narita, A., Maeda, Y., Honda, H., Fujiwara, I.(2025) Proc Natl Acad Sci U S A 122: e2502164122-e2502164122
- PubMed: 40658853
- DOI: https://doi.org/10.1073/pnas.2502164122
- Primary Citation of Related Structures:
9L2N - PubMed Abstract:
Cytochalasin D (CytoD), a widely used actin inhibitor, is typically employed in cell studies as a simple barbed end capper. However, accumulating evidence suggests broader effects on actin dynamics. We addressed this by observing single actin filaments using total internal reflection fluorescence microscopy. Our depolymerization assay confirmed that, at nanomolar concentrations, CytoD tightly caps barbed ends. The K 1/2 for inhibition was 4.1 nM, consistent with previous bulk measurements, and our approach revealed a capping duration of ~2 min. In polymerization assays, nanomolar CytoD completely suppressed barbed end elongation. Interestingly, at subnanomolar concentrations, CytoD caps barbed ends only transiently, rapidly associating and dissociating under both polymerizing and depolymerizing conditions. We interpreted this contradictory behavior as arising from differences in binding modes: capping one strand (fast dissociation) or both strands (slow dissociation). CytoD severs actin filaments at micromolar levels, a concentration range commonly used in cell biological studies. Although the severing rate is slower than cofilin, its higher frequency leads to filament fragmentation. Severing activity was suppressed by inorganic phosphate or cofilin. Our crystal structure of CytoD bound to filamentous conformation (F-form) actin showed that CytoD fits better in the hydrophobic cleft of F-form actin than monomeric conformation actin, explaining its preference for barbed end subunits. CytoD prevents barbed end depolymerization by stabilizing the terminal subunits in the F-form, which is supported by our MD simulations. These findings reveal the molecular mechanisms by which CytoD modulates actin dynamics and highlight the need for careful dosage control when treating cells with CytoD.
- Department of Materials Sciences and Bioengineering, Nagaoka University of Technology, Nagaoka, Niigata 940-2188, Japan.
Organizational Affiliation: