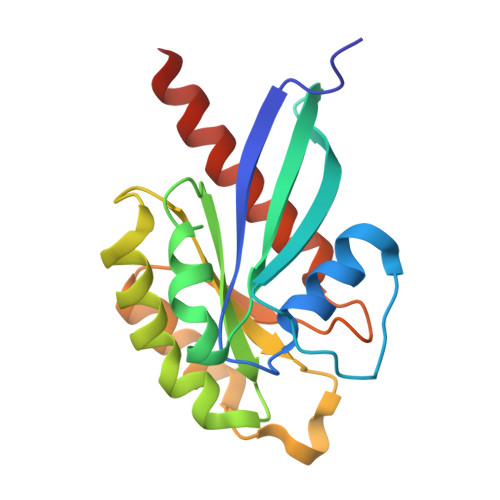
Functional and Structural Insights Into Complex Formation Between OPTN Leucine Zipper Domain and RAB8A.
Okatsu, K., Kikuchi, R., Matsuda, N., Fukai, S., Yamano, K.(2025) Genes Cells 30: e70043-e70043
- PubMed: 40770829
- DOI: https://doi.org/10.1111/gtc.70043
- Primary Citation of Related Structures:
9M0O - PubMed Abstract:
Optineurin (OPTN) is a multifunctional adaptor protein involved in vesicular trafficking and selective autophagy. In this study, we investigated the molecular mechanism by which OPTN regulates these distinct processes through the leucine zipper (LZ) domain. OPTN interacts with the active form of RAB8A and closely related RAB proteins (RAB8B and RAB10). We determined the crystal structure of the OPTN-RAB8A complex at 1.83 Å resolution and elucidated the specific interaction mechanism between these proteins. Structure-guided mutational analysis at the molecular and cellular level suggested that OPTN interacts with RAB8A on two distinct surfaces. RAB8A-interacting surfaces of OPTN include residues that are located apart from the LZ-forming region. Furthermore, the interaction between OPTN and RAB8A was corroborated by cell biological approaches. Although RAB8A/8B/10 were not essential for mitophagy in experiments using their triple knockout cells, the RAB8A-binding residues of OPTN were critical for the recruitment of ATG9A vesicles. Therefore, our results provide molecular insights into the functional role of the LZ domain of OPTN in regulating vesicular trafficking and selective autophagy.
- Department of Chemistry, Graduate School of Science, Kyoto University, Kyoto, Japan.
Organizational Affiliation: