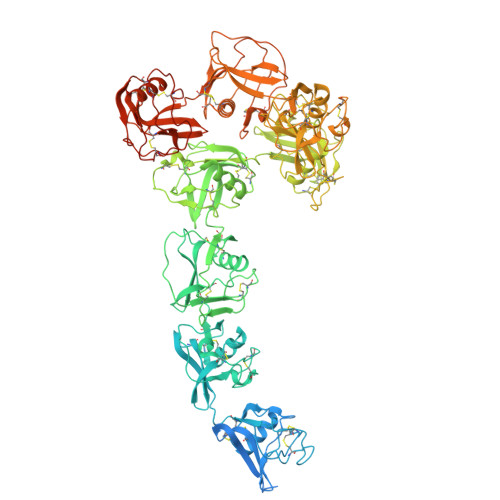
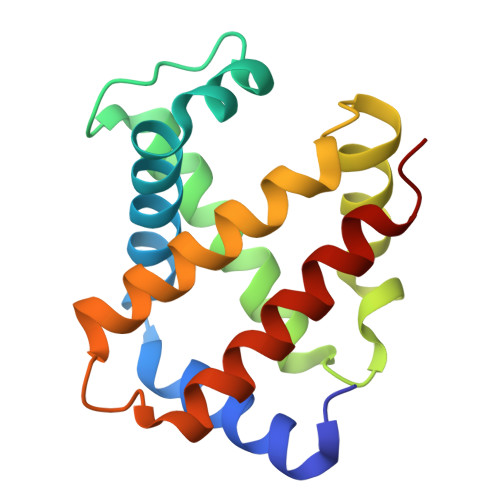
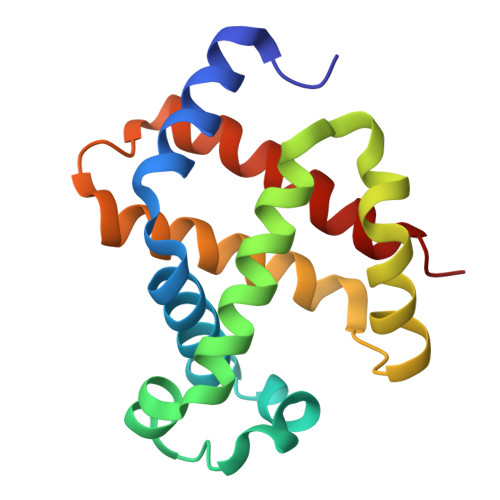
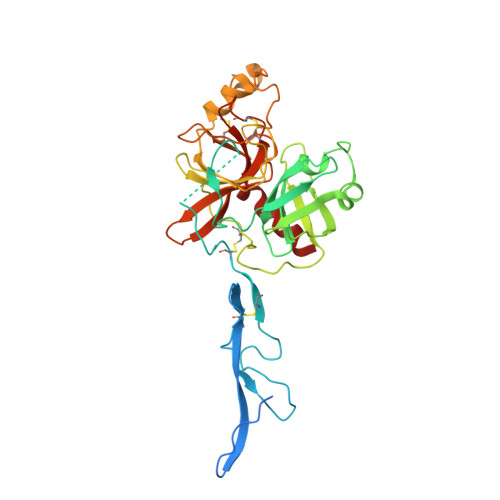
Structural elucidation of the haptoglobin-hemoglobin clearance mechanism by macrophage scavenger receptor CD163.
Huang, C.S., Wang, H., White, J.B.R., Degtjarik, O., Huynh, C., Brannstrom, K., Horn, M.T., Muench, S.P., Somers, W.S., Chaparro-Riggers, J., Lin, L., Mosyak, L.(2025) PLoS Biol 23: e3003264-e3003264
- PubMed: 40644526
- DOI: https://doi.org/10.1371/journal.pbio.3003264
- Primary Citation of Related Structures:
9NB5, 9NB6, 9NB8 - PubMed Abstract:
Intravascular hemolysis releases hemoglobin into the bloodstream, which can damage vascular and renal tissues due to its oxidative nature. Circulating haptoglobin acts as a primary defense by binding to free hemoglobin, forming a haptoglobin-hemoglobin (HpHb) complex that is then recognized and cleared by the CD163 scavenger receptor on macrophages. While the function and structure of HpHb complex are mostly well-defined, the molecular mechanism underlying its interaction with CD163 remains unclear. Here we report the cryo-electron microscopy structures of human CD163 in its unliganded state and in its complex with HpHb. These structures reveal that CD163 functions as a trimer, forming a composite binding site at its center for one protomer of the dimeric HpHb, resulting in a 3:1 binding stoichiometry. In the unliganded state, CD163 can also form a trimer, but in an autoinhibitory configuration that occludes the ligand binding site. Widespread electrostatic interactions mediated by calcium ions are pivotal in both pre-ligand and ligand-bound receptor assemblies. This calcium-dependent mechanism enables CD163/HpHb complexes to assemble and, once internalized, disassemble into individual components upon reaching the endosome, where low calcium and lower pH conditions prevail. Collectively, this study elucidates the molecular mechanism by which CD163-mediated endocytosis efficiently clears different isoforms of HpHb.
- BioMedicine Design, Pfizer, Inc., Cambridge, Massachusetts, United States of America.
Organizational Affiliation: