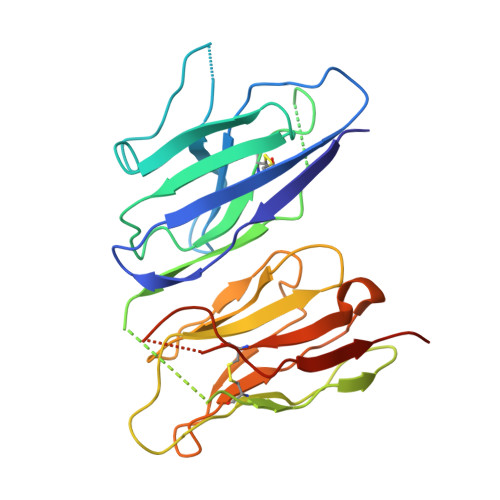
Structure-Guided Stapling of Dimeric Conformations and Linker Engineering Enhance Thermostability and Fine-Tune Activity of Bispecific VHH Cytokine Agonists.
Trenker, R., Rokkam, D., Morin, A., Balasubrahmanyam, P., Paredes, V., Cheng, I., de Waal Malefyt, R., Oft, M., Lupardus, P., Vivona, S.(2025) Antibodies (Basel) 14
- PubMed: 40981273
- DOI: https://doi.org/10.3390/antib14030074
- Primary Citation of Related Structures:
9O99, 9O9A, 9O9B, 9O9C - PubMed Abstract:
Bispecific antibodies have emerged as a promising class of therapeutics, enabling simultaneous targeting of two distinct antigens. Single-domain antibodies (sdAbs) comprising camelid variable heavy chains (VHHs) provide a compact and adaptable platform for bispecific antibody design due to their small size and ease of linkage. Here we investigate structure-activity relationship of VHH-based cytokine surrogates by combining cell signaling and functional assays with x-ray crystallography and other biophysical techniques. We describe crystal structures of four unique bispecific VHHs that engage and activate the cytokine receptor pairs IL-18Rα/IL-18Rβ and IL-2Rβ/IL-2Rγ. These bispecific VHH molecules, referred to as surrogate cytokine agonists (SCAs), create unique cytokine signals that can be tuned by linker engineering. Our structural analysis reveals multiple dimeric conformations for these bispecific SCAs, where the two VHH domains can interact to form a compact structure. We demonstrate that the dimeric conformation can be enforced via engineering of a non-native disulfide bond between the VHH subunits, thus enhancing molecular thermostability. Our findings have important implications for the design and engineering of bispecific VHHs or sdAbs, offering a novel strategy for tuning their activity and increasing their stability.
- Department of Discovery, Pharmacology and Translational Research, Synthekine, Menlo Park, CA 94025, USA.
Organizational Affiliation: