Overcoming air-water interface-induced artifacts in Cryo-EM with protein nanocrates
Jenkins, M.C., Bobe, D., Johnston, J.D., Cheung, J., Karasawa, A., Zimanyi, C.M., Dermanci, O., Finn, M.G., de Marco, A., Kopylov, M.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Capsid protein | 129 | Escherichia phage MS2 | Mutation(s): 0 | 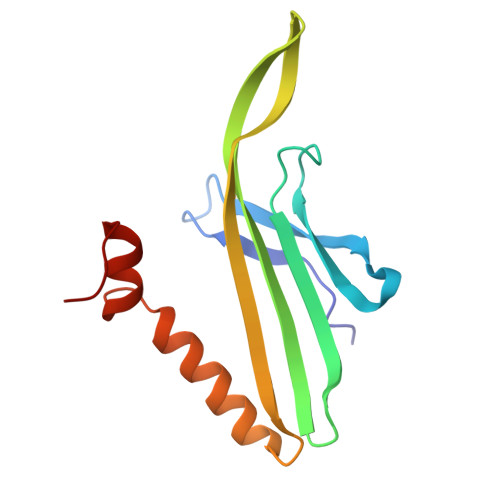 | |
UniProt | |||||
Find proteins for P03612 (Escherichia phage MS2) Explore P03612 Go to UniProtKB: P03612 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P03612 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Task | Software Package | Version |
|---|---|---|
| RECONSTRUCTION | cryoSPARC | |
| MODEL REFINEMENT | PHENIX | 1.21.1_5286 |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Simons Foundation | United States | SF349247 |
| National Institutes of Health/National Institute Of Allergy and Infectious Diseases (NIH/NIAID) | United States | R01 AI148382 |
| National Institutes of Health/National Cancer Institute (NIH/NCI) | United States | R01 CA247484 |