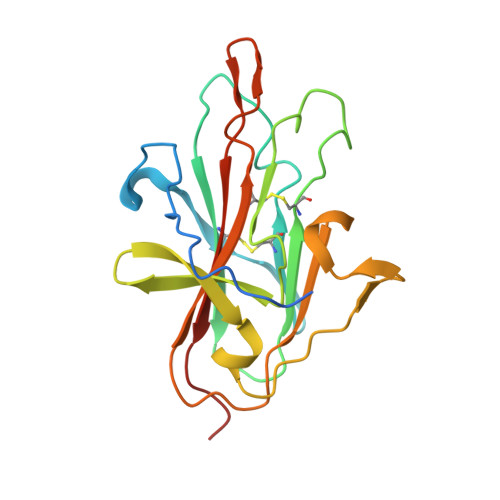
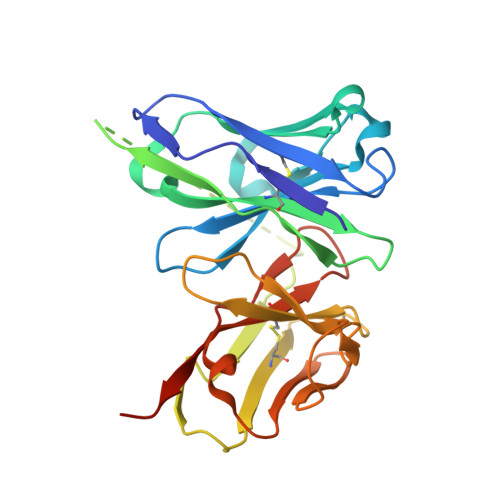
A human monoclonal antibody blocks malaria transmission and defines a highly conserved neutralizing epitope on gametes.
Coelho, C.H., Tang, W.K., Burkhardt, M., Galson, J.D., Muratova, O., Salinas, N.D., Alves E Silva, T.L., Reiter, K., MacDonald, N.J., Nguyen, V., Herrera, R., Shimp, R., Narum, D.L., Byrne-Steele, M., Pan, W., Hou, X., Brown, B., Eisenhower, M., Han, J., Jenkins, B.J., Doritchamou, J.Y.A., Smelkinson, M.G., Vega-Rodriguez, J., Truck, J., Taylor, J.J., Sagara, I., Renn, J.P., Tolia, N.H., Duffy, P.E.(2021) Nat Commun 12: 1750-1750
- PubMed: 33741942
- DOI: https://doi.org/10.1038/s41467-021-21955-1
- Primary Citation of Related Structures:
7JUM - PubMed Abstract:
Malaria elimination requires tools that interrupt parasite transmission. Here, we characterize B cell receptor responses among Malian adults vaccinated against the first domain of the cysteine-rich 230 kDa gamete surface protein Pfs230, a key protein in sexual stage development of P. falciparum parasites. Among nine Pfs230 human monoclonal antibodies (mAbs) that we generated, one potently blocks transmission to mosquitoes in a complement-dependent manner and reacts to the gamete surface; the other eight show only low or no blocking activity. The structure of the transmission-blocking mAb in complex with vaccine antigen reveals a large discontinuous conformational epitope, specific to domain 1 of Pfs230 and comprising six structural elements in the protein. The epitope is conserved, suggesting the transmission-blocking mAb is broadly functional. This study provides a rational basis to improve malaria vaccines and develop therapeutic antibodies for malaria elimination.
- Pathogenesis and Immunity Section, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Organizational Affiliation: