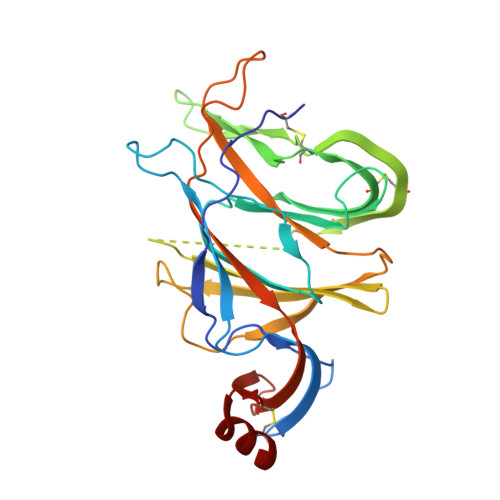
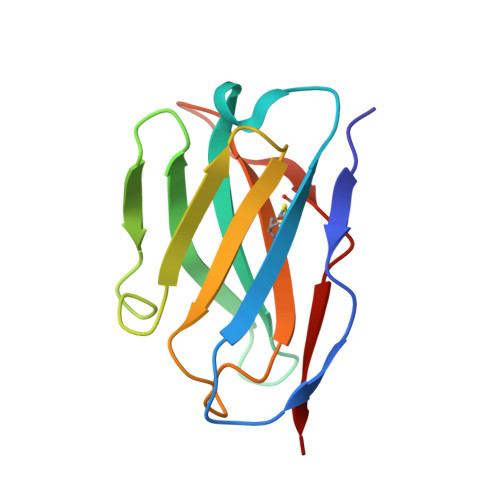
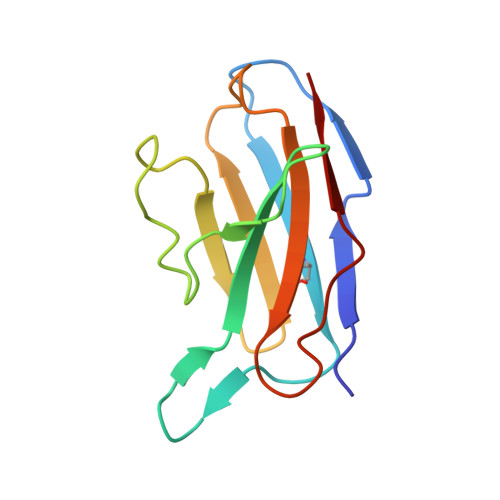
A broadly reactive antibody targeting the N-terminal domain of SARS-CoV-2 spike confers Fc-mediated protection.
Adams, L.J., VanBlargan, L.A., Liu, Z., Gilchuk, P., Zhao, H., Chen, R.E., Raju, S., Chong, Z., Whitener, B.M., Shrihari, S., Jethva, P.N., Gross, M.L., Crowe Jr., J.E., Whelan, S.P.J., Diamond, M.S., Fremont, D.H.(2023) Cell Rep Med 4: 101305-101305
- PubMed: 38039973
- DOI: https://doi.org/10.1016/j.xcrm.2023.101305
- Primary Citation of Related Structures:
7SWW, 7SWX - PubMed Abstract:
Most neutralizing anti-SARS-CoV-2 monoclonal antibodies (mAbs) target the receptor binding domain (RBD) of the spike (S) protein. Here, we characterize a panel of mAbs targeting the N-terminal domain (NTD) or other non-RBD epitopes of S. A subset of NTD mAbs inhibits SARS-CoV-2 entry at a post-attachment step and avidly binds the surface of infected cells. One neutralizing NTD mAb, SARS2-57, protects K18-hACE2 mice against SARS-CoV-2 infection in an Fc-dependent manner. Structural analysis demonstrates that SARS2-57 engages an antigenic supersite that is remodeled by deletions common to emerging variants. In neutralization escape studies with SARS2-57, this NTD site accumulates mutations, including a similar deletion, but the addition of an anti-RBD mAb prevents such escape. Thus, our study highlights a common strategy of immune evasion by SARS-CoV-2 variants and how targeting spatially distinct epitopes, including those in the NTD, may limit such escape.
- Department of Pathology and Immunology, Washington University School of Medicine, St. Louis, MO 63110, USA.
Organizational Affiliation: