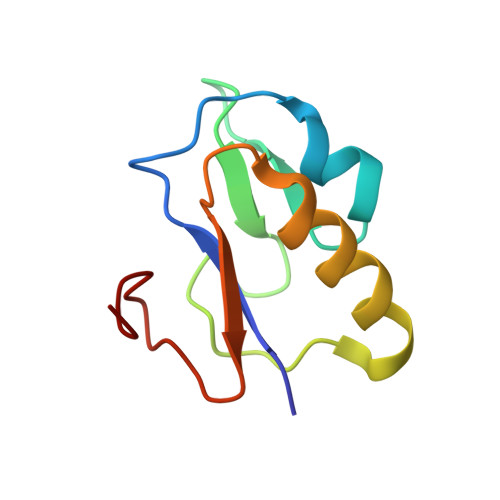
Protonation/deprotonation-driven switch for the redox stability of the low-potential [4Fe-4S] ferredoxin
Wada, K., Kobayashi, K., Era, I., Isobe, Y., Kamimura, T., Marukawa, M., Nagae, T., Honjo, K., Kaseda, N., Motoyama, Y., Inoue, K., Sugishima, M., Kusaka, K., Yano, N., Fukuyama, K., Mishima, M., Kitagawa, Y., Unno, M.(2025) Elife