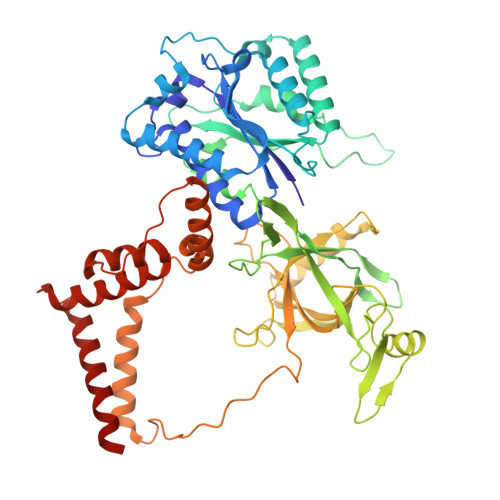
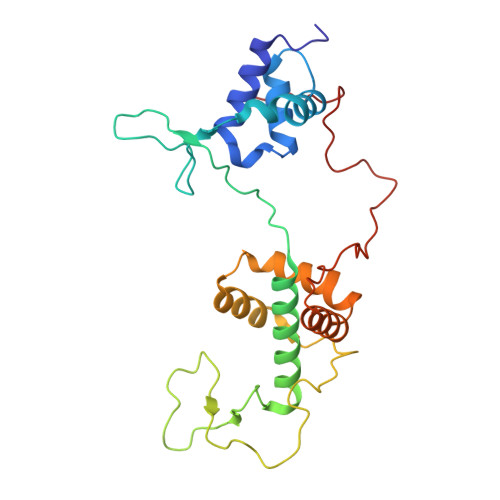
Restriction of Ku translocation protects telomere ends.
Mattarocci, S., Baconnais, S., Roisne-Hamelin, F., Pobiega, S., Alibert, O., Morin, V., Deshayes, A., Veaute, X., Ropars, V., Chevreuil, M., Mehringer, J., Busso, D., Mazon, G., Fernandez Varela, P., Le Cam, E., Charbonnier, J.B., Cuniasse, P., Marcand, S.(2025) Nat Commun 16: 6824-6824
- PubMed: 40707444
- DOI: https://doi.org/10.1038/s41467-025-61864-1
- Primary Citation of Related Structures:
8S82, 8S8P - PubMed Abstract:
Safeguarding chromosome ends against fusions via nonhomologous end joining (NHEJ) is essential for genome integrity. Paradoxically, the conserved NHEJ core factor Ku binds telomere ends. How it is prevented from promoting NHEJ remains unclear, as does the mechanism that allows Ku to coexist with telomere-protective DNA binding proteins, Rap1 in Saccharomyces cerevisiae. Here, we find that Rap1 directly inhibits Ku's NHEJ function at telomeres. A single Rap1 molecule near a double-stand break suppresses NHEJ without displacing Ku in cells. Furthermore, Rap1 and Ku form a complex on short DNA duplexes in vitro. Cryo-EM shows Rap1 blocks Ku's inward translocation on DNA - an essential step for NHEJ at DSBs. Nanopore sequencing of telomere fusions confirms this mechanism protects native telomere ends. These findings uncover a telomere protection mechanism where Rap1 restricts Ku's inward translocation. This switches Ku from a repair-promoting to a protective role preventing NHEJ at telomeres.
- Université Paris-Saclay, Université Paris Cité, CEA, Inserm, Institut de biologie François Jacob, UMR Stabilité Génétique Cellules Souches et Radiations, Fontenay-aux-Roses, France. stefano.mattarocci@inserm.fr.
Organizational Affiliation: