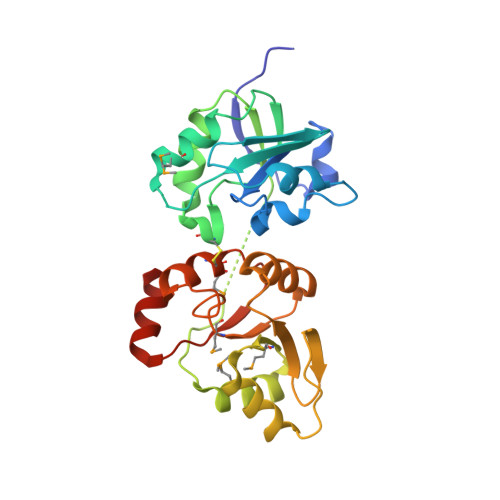
Torsional twist of the SARS-CoV and SARS-CoV-2 SUD-N and SUD-M domains.
Rosas-Lemus, M., Minasov, G., Brunzelle, J.S., Taha, T.Y., Lemak, S., Yin, S., Shuvalova, L., Rosecrans, J., Khanna, K., Seifert, H.S., Savchenko, A., Stogios, P.J., Ott, M., Satchell, K.J.F.(2025) Protein Sci 34: e70050-e70050
- PubMed: 39969084
- DOI: https://doi.org/10.1002/pro.70050
- Primary Citation of Related Structures:
8UFL, 8UFM - PubMed Abstract:
Coronavirus non-structural protein 3 (nsp3) forms hexameric crowns of pores in the double membrane vesicle that houses the replication-transcription complex. Nsp3 in SARS-like viruses has three unique domains absent in other coronavirus nsp3 proteins. Two of these, SUD-N (Macrodomain 2) and SUD-M (Macrodomain 3), form two lobes connected by a peptide linker and an interdomain disulfide bridge. We resolve the first complete x-ray structure of SARS-CoV SUD-N/M as well as a mutant variant of SARS-CoV-2 SUD-N/M modified to restore cysteines for interdomain disulfide bond naturally lost by evolution. Comparative analysis of all structures revealed SUD-N and SUD-M are not rigidly associated but rather have significant rotational flexibility. Phylogenetic analysis supports that the potential to form the disulfide bond is common across betacoronavirus isolates from many bat species and civets, but also one or both of the cysteines that form the disulfide bond are absent across isolates from bats and pangolins. The absence of these cysteines does not impact viral replication or protein translation.
- Department of Microbiology-Immunology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA.
Organizational Affiliation: