A novel antidepressant acting via allosteric inhibition of GluN2D-incorporated NMDA receptors at GABAergic interneurons
Zhang, J.L., Zhu, S.J., Fei, G.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Isoform 6 of Glutamate receptor ionotropic, NMDA 1 | 868 | Homo sapiens | Mutation(s): 0 Gene Names: GRIN1, NMDAR1 | 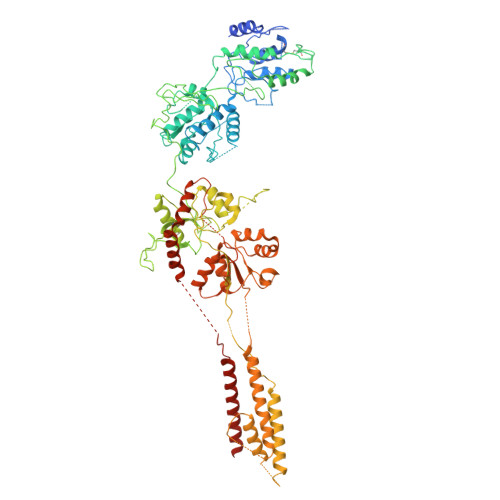 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q05586 (Homo sapiens) Explore Q05586 Go to UniProtKB: Q05586 | |||||
PHAROS: Q05586 GTEx: ENSG00000176884 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q05586 | ||||
Glycosylation | |||||
| Glycosylation Sites: 2 | Go to GlyGen: Q05586-1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Glutamate receptor ionotropic, NMDA 2D | 911 | Homo sapiens | Mutation(s): 0 Gene Names: GRIN2D, GluN2D, NMDAR2D | 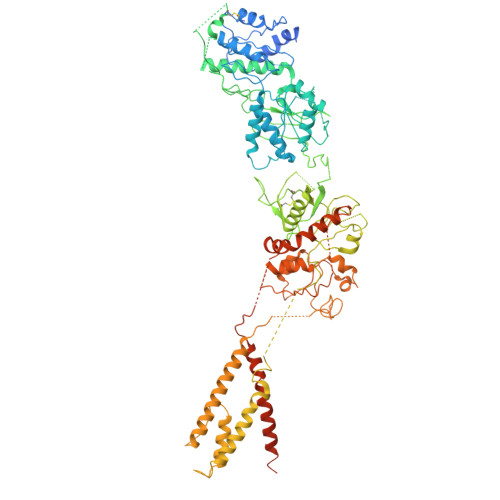 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O15399 (Homo sapiens) Explore O15399 Go to UniProtKB: O15399 | |||||
PHAROS: O15399 GTEx: ENSG00000105464 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O15399 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| A1LW8 (Subject of Investigation/LOI) Query on A1LW8 | G [auth B], J [auth D] | (2~{R},3~{S},4~{S},5~{R},6~{R})-2-(hydroxymethyl)-6-[(2~{S})-2-methyl-4-[(1~{R},2~{R},4~{S},6~{S},7~{S},8~{R},9~{S},12~{S},13~{R},16~{S},18~{R})-7,9,13,18-tetramethyl-16-oxidanyl-5-oxapentacyclo[10.8.0.0^{2,9}.0^{4,8}.0^{13,18}]icosan-6-yl]butoxy]oxane-3,4,5-triol C34 H56 O8 ULNDCEKFZLEEGC-TWIDTKOTSA-N |  | ||
| NAG Query on NAG | E [auth A], F [auth A], H [auth C], I [auth C] | 2-acetamido-2-deoxy-beta-D-glucopyranose C8 H15 N O6 OVRNDRQMDRJTHS-FMDGEEDCSA-N |  | ||
| Task | Software Package | Version |
|---|---|---|
| RECONSTRUCTION | RELION | 3.1.1 |
| MODEL REFINEMENT | PHENIX | 1.20.1 |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Natural Science Foundation of China (NSFC) | China | 31771115 |
| National Natural Science Foundation of China (NSFC) | China | 31671049 |
| National Natural Science Foundation of China (NSFC) | China | 81030065 |
| Chinese Academy of Sciences | China | XDB32020000 |
| Chinese Academy of Sciences | China | XDA12040214 |
| Chinese Academy of Sciences | China | XDA12040220 |
| National Natural Science Foundation of China (NSFC) | China | 81803828 |
| Chinese Academy of Sciences | China | 2019280 |
| National Natural Science Foundation of China (NSFC) | China | 32221003 |