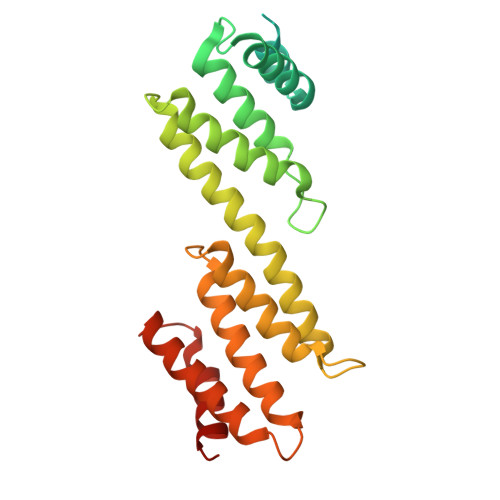
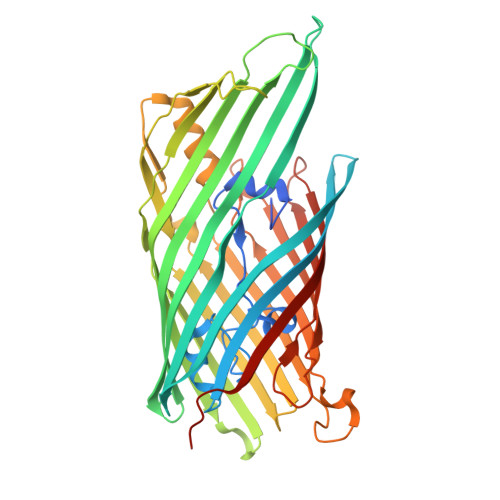
Structure of a distinct beta-barrel assembly machinery complex in the Bacteroidota.
Silale, A., Madej, M., Mikruta, K., Frey, A.M., Hart, A.J., Basle, A., Scavenius, C., Enghild, J.J., Trost, M., Hirt, R.P., van den Berg, B.(2025) Nat Microbiol 10: 2845-2859
- PubMed: 41034344
- DOI: https://doi.org/10.1038/s41564-025-02132-2
- Primary Citation of Related Structures:
9HIS, 9HIV, 9HJ3, 9HJM - PubMed Abstract:
The Gram-negative β-barrel assembly machinery (BAM) complex catalyses the folding and membrane insertion of newly synthesized β-barrel outer membrane proteins. The BAM is structurally conserved, but most studies have focused on Gammaproteobacteria. Here, using single-particle cryogenic electron microscopy, quantitative proteomics and functional assays, we show that the BAM complex is distinct within the Bacteroidota. Cryogenic electron microscopy structures of BAM complexes from the human gut symbiont Bacteroides thetaiotaomicron (3.3 Å) and the human oral pathogen Porphyromonas gingivalis (3.2 Å) show similar, seven-component complexes of ~325 kDa. The complexes are mostly extracellular and comprise canonical BamA and BamD; an integral, essential outer membrane protein, BamG, that associates with BamA; and four surface-exposed lipoproteins: BamH-K. Absent from the BAM in Pseudomonadota, BamG-K form a large, extracellular dome that may confer additional functionality to enable the folding and assembly of β-barrel-surface-exposed lipoprotein complexes that are a hallmark of the Bacteroidota. Our findings develop our understanding of fundamental biological processes in an important bacterial phylum.
- Biosciences Institute, Faculty of Medical Sciences, Newcastle University, Newcastle upon Tyne, UK.
Organizational Affiliation: