Structural analysis of full-length human transmembrane protein 94 argues against its classification as a P-type Mg 2+ ATPase.
Li, Y., Cong, Y., Lou, X., Li, W., Wang, R., Gong, M., Xiao, J., Qian, D., Yan, C., Gong, D.(2025) Cell Discov 11: 54-54
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Endoplasmic reticulum magnesium-transporting P-type ATPase | 1,394 | Homo sapiens | Mutation(s): 0 Gene Names: TMEM94, KIAA0195 EC: 7.2.2.14 Membrane Entity: Yes | 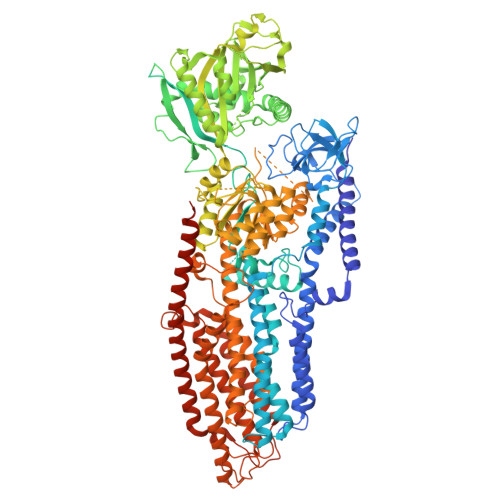 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q12767 (Homo sapiens) Explore Q12767 Go to UniProtKB: Q12767 | |||||
PHAROS: Q12767 GTEx: ENSG00000177728 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q12767 | ||||
Glycosylation | |||||
| Glycosylation Sites: 2 | Go to GlyGen: Q12767-1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| POV (Subject of Investigation/LOI) Query on POV | D [auth A] | (2S)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propyl 2-(trimethylammonio)ethyl phosphate C42 H82 N O8 P WTJKGGKOPKCXLL-PFDVCBLKSA-N |  | ||
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | 1.20.1_4487: |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Not funded | -- |