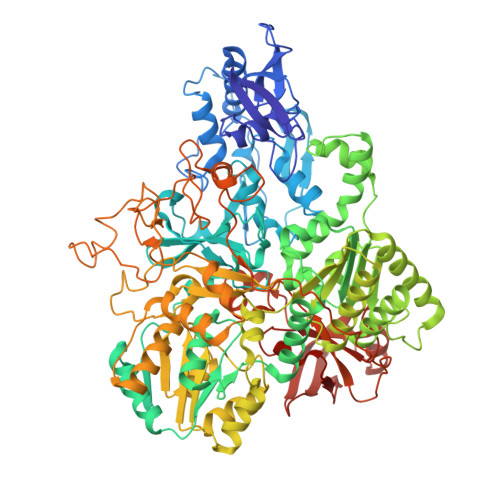
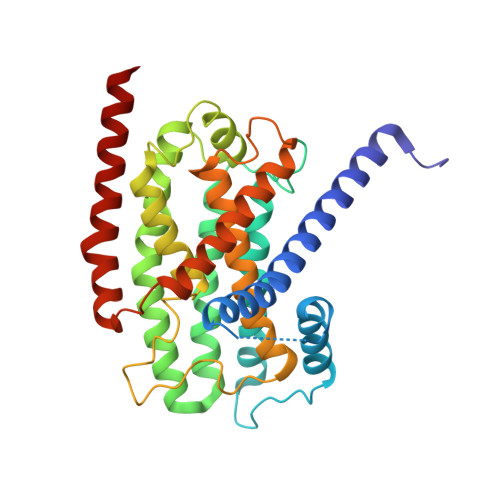
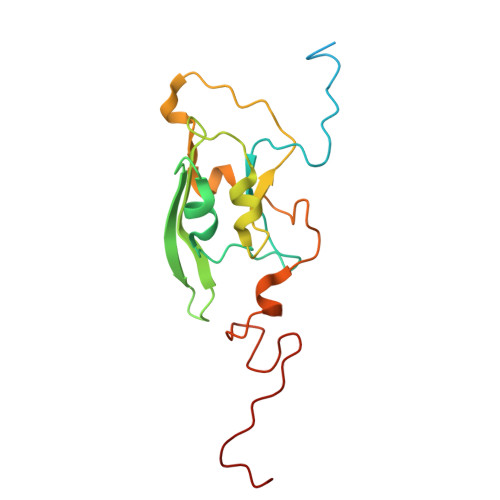
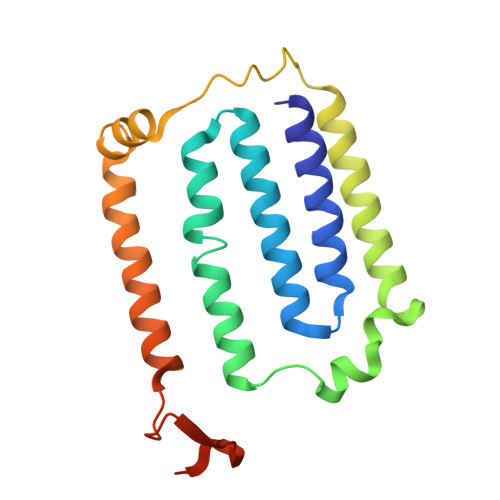
Structural changes shifting the redox potential of the outlying cluster N1a in respiratory complex I.
Wohlwend, D., Seifermann, T., Gnandt, E., Vranas, M., Gerhardt, S., Friedrich, T.(2025) Structure
- PubMed: 41265450
- DOI: https://doi.org/10.1016/j.str.2025.10.016
- Primary Citation of Related Structures:
9HE5, 9HEG, 9HEM, 9HEN, 9Q8I - PubMed Abstract:
Energy-converting NADH:ubiquinone oxidoreductase, respiratory complex I, is central to energy metabolism by coupling NADH oxidation and quinone reduction with proton translocation across the membrane. Electrons are transferred from the primary acceptor flavin mononucleotide via a chain of iron-sulfur clusters to quinone. The enigmatic cluster N1a is conserved, but not part of this electron transfer chain. We reported on variants of the complex in which N1a is not detectable by EPR spectroscopy. This was tentatively attributed to the lower redox potential of the variant N1a. However, it remained an open question, whether the variants contain this cluster at all. Here, we determined the structures of these variants by X-ray crystallography and cryogenic-electron microscopy. Cluster N1a is present in all variants and the shift of its redox potential is explained by nearby structural changes. A role of the cluster for the mechanism of the complex is discussed.
- Institut für Biochemie, Albert-Ludwigs-Universität Freiburg, Freiburg im Breisgau, Germany.
Organizational Affiliation: