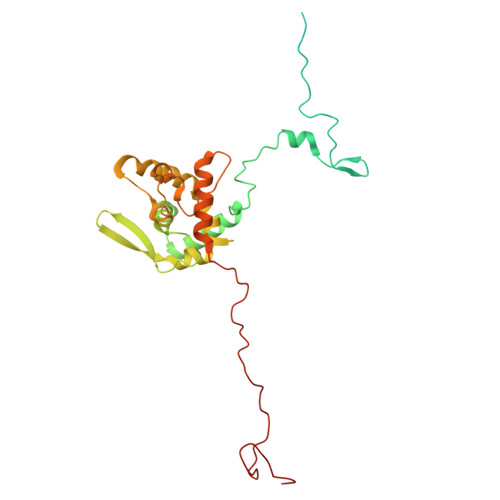
Species-specific structural adaptation of the potyviral coat protein in virions and virus-like particles.
Koritnik, N., Kezar, A., Kavcic, L., Znidaric, M.T., Leonardi, A., De, S., Pollari, M., Makinen, K., Podobnik, M.(2026) Commun Biol
- PubMed: 41530503
- DOI: https://doi.org/10.1038/s42003-025-09502-w
- Primary Citation of Related Structures:
9R7R, 9R7S, 9R7T, 9R7U, 9R7V, 9R7X, 9R7Y, 9R7Z, 9R80, 9R81, 9R9W, 9R9X, 9R9Y, 9R9Z, 9RA0, 9RA1, 9RA2 - PubMed Abstract:
Potyviruses are the largest group of plant positive-sense single-stranded RNA viruses and represent a major economic burden worldwide. Their coat protein (CP) forms a filamentous, flexible capsid around the genomic RNA. However, information is still lacking on the mechanisms of virion assembly, disassembly and stability, which is central to understanding virus biology and control. Here, we investigate the role of CP in these processes using structural, biochemical and biophysical studies of five potyviral CPs from three phylogenetic clades combined with bioinformatics and in planta experiments. Our results suggest that, while potyviruses have a conserved virion structure, the amino acids forming the CP-CP and CP-RNA interactions leading to this structure are species-specific. We show that the species-specific CP sequence also determines the architecture of RNA-free virus-like particles (VLPs) and the degree of their structural polymorphism. We identify the residues that determine this specificity at distinct S1-S4 interaction sites. In contrast, a highly conserved charged amino acid triad at the CP-CP interface is essential for the stability of virions and RNA-free VLPs. These results contribute to understanding the molecular mechanism of potyviral virion assembly and highlight the significance of the amino acid sequence of selected CPs in potential biotechnological or biomedical applications.
- Department of Molecular Biology and Nanobiotechnology, National Institute of Chemistry, Ljubljana, Slovenia.
Organizational Affiliation: